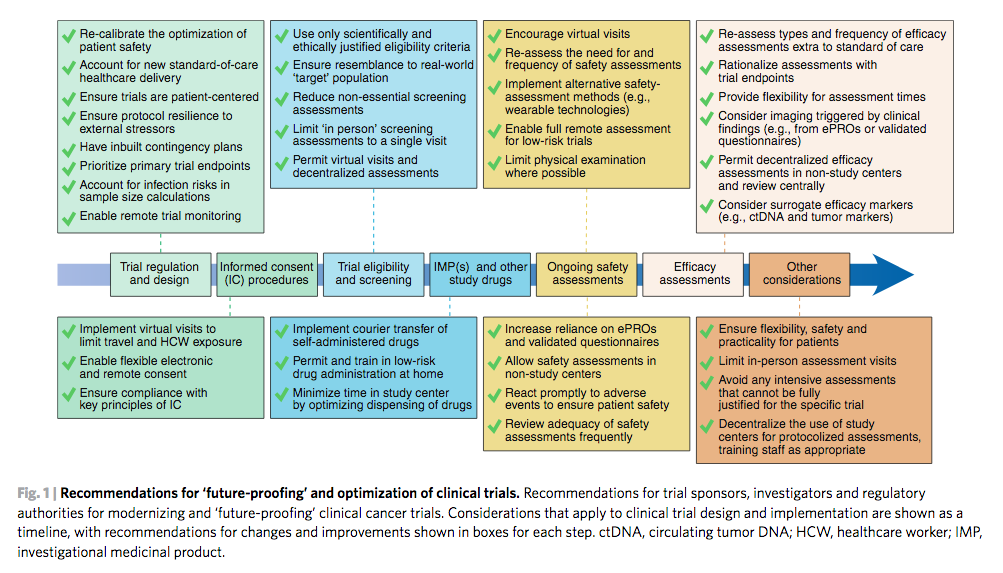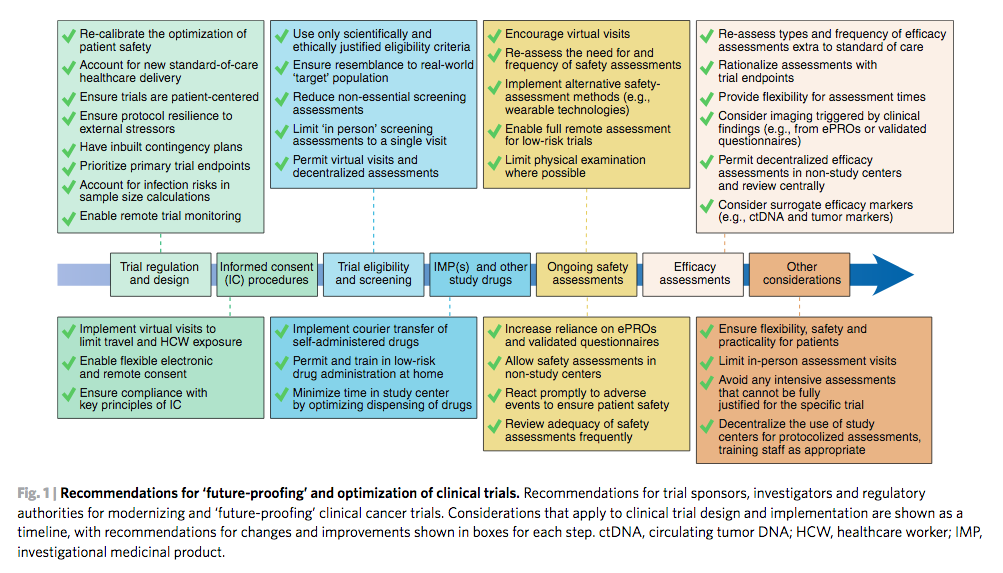
Despite the importance of clinical trials for delivering new medicines to benefit patients, in regions affected by COVID-19 many cancer trials have stopped enrolling new patients and new trials have not been able to open, profoundly hampering innovation.
The current situation has forced a critical re-evaluation of the traditional processes of clinical research, including how these may pose particular infection risks to cancer patients, and how competing risks can be mitigated to build more resilient research.
Many of the adaptations to normal clinical practice that have been embraced swiftly by clinicians during the pandemic (such as telephone/video consultations) are likely to persist in the longer term, with significant implications for clinical research.
Gary Doherty (Consultant Medical Oncologist at Addenbrooke’s and member of the Aerodigestive Cancer and Neuro-Oncology Programmes), along with a fifth year medical student (Mehmet Goksu) and a clinical trial fellow (Bruno de Paula) have used these observations and their own experience to reconsider the conduct of clinical cancer research.
Their paper, published in Nature Cancer, offers advice on how to deliver clinical trials safely and efficiently during, and beyond, the current outbreak. They discuss ways to modernise clinical trials and improve their patient focus, while also ‘future-proofing’ them against further waves of COVID-19 infection or potential new threats.
The recommendations can be used either to adapt ongoing clinical trials, or to help design and implement new clinical research studies. By breaking down every stage of the clinical trial process from enrolling patients, screening assessments, delivering trial treatment, safety assessments and measuring the effectiveness of treatment, the authors evaluate current standard procedures and suggest new patient-centred adaptations that will promote patient safety and trial integrity.
The authors also discuss contingency measures for COVID-19 itself, as well as how to protect patients and trials against this specific threat, and conclude by emphasising how clinical research must take particular note of how COVID-19 has changed standard healthcare delivery and risk management, and learn from this.
Speaking about this work, Dr Doherty said: “With every challenge comes an opportunity. While COVID-19 has temporarily decimated clinical cancer research, it has also exposed its poor resilience and poor patient-centeredness. A long overdue, critical re-evaluation of clinical cancer research is necessary to improve these aspects, and we believe our work lays the groundwork for how this can be achieved practically.
Reference:
Gary J. Doherty, Mehmet Goksu & Bruno H. R. de Paula Rethinking cancer clinical trials for COVID-19 and beyond Nature Cancer https://doi.org/10.1038/s43018-020-0083-x