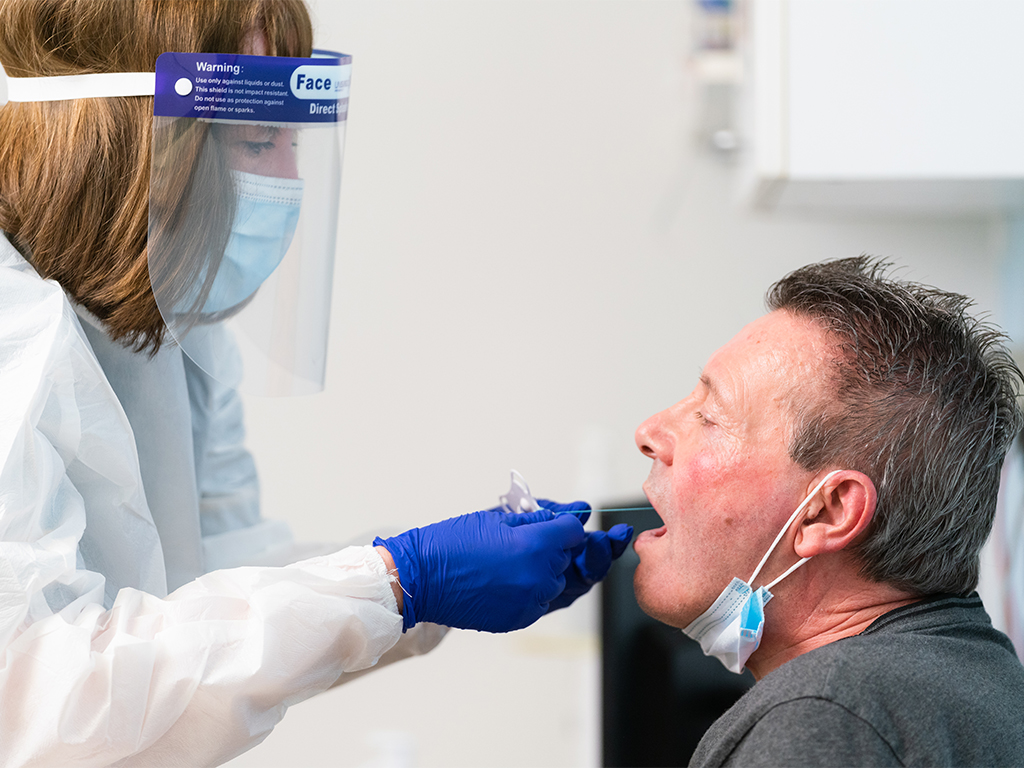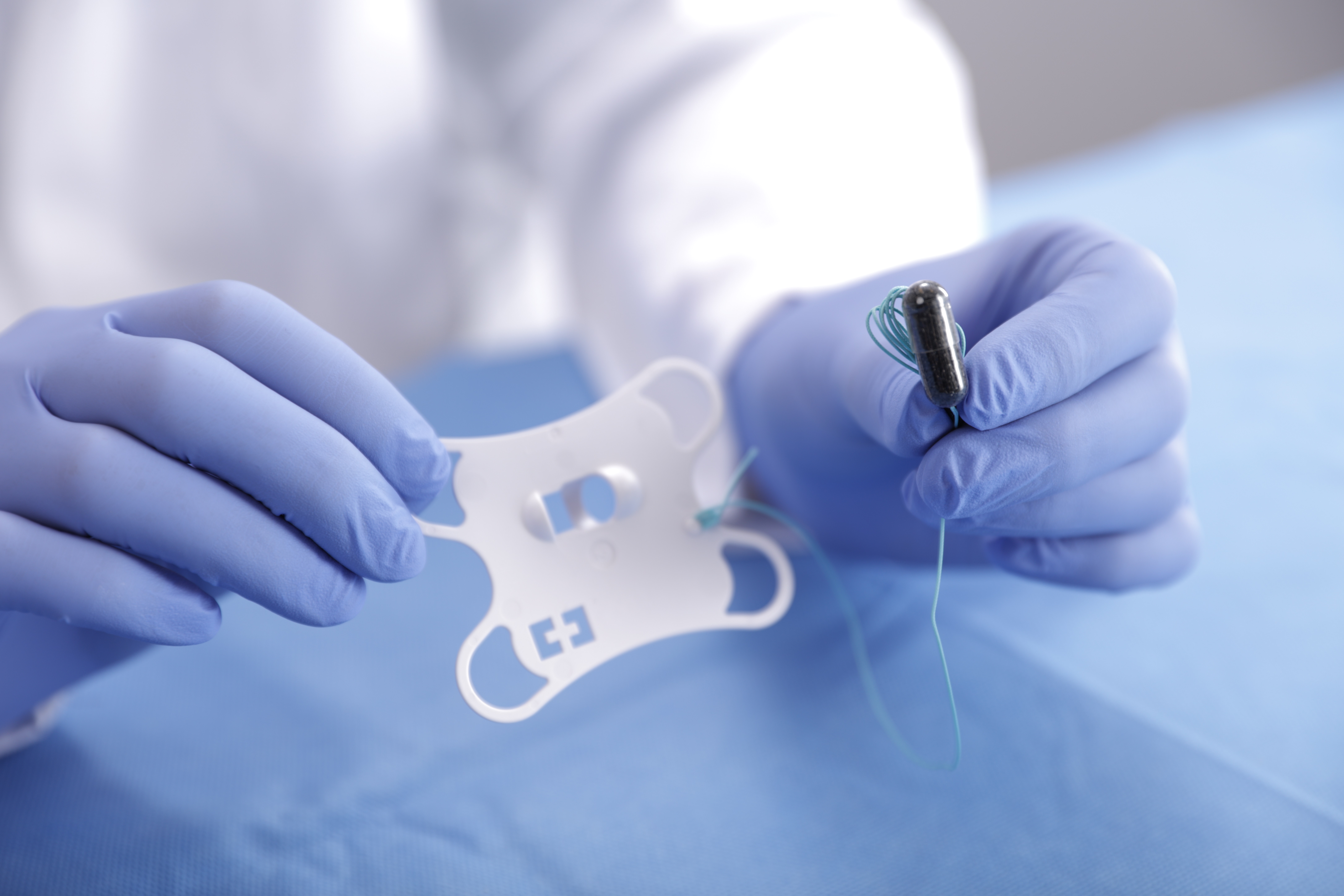
Scotland is the first country in the world to implement the use of the Cytosponge across all mainland Scottish health boards in a national screening programme.
The innovative ‘sponge on a string’ device was invented and developed by Professor Rebecca Fitzgerald, who co-leads our Early Detection Programme, and her team at the University of Cambridge and Addenbrooke’s Hospital.
The Cytosponge, manufactured by Medtronic, is non-aerosol generating, non-invasive and does not require sedation. In just 10 minutes, Barrett’s Oesophagus patients can be screened for oesophageal adenocarcinoma (OAC) or its pre-curser dysplasia.
It has been fast tracked into clinical practice to help manage risk for healthcare professionals and vulnerable patients during the Covid-19 pandemic and beyond.
Professor Fitzgerald said: “We’ve trained the nurses across all the health boards in Scotland who are widely adopting and implementing this test.
"The samples are sent down to the Cyted laboratories for analysis and the results are got back to the patients within a couple of weeks.”
Early detection of OAC can improve patient survival dramatically; treated at stage 1; OAC has a survival rate greater than 90%.
Scotland’s world-first screening programme has already had life-saving outcomes: successfully identifying cancerous cells in 12 patients in Scotland during COVID-19.
In Scotland, the diagnostic pathway was significantly slowed down by the pandemic. There are now increased numbers of patients waiting for a diagnostic Upper GI endoscopy, with some patients experiencing delays of over a year.
In addition, patients with known Barrett’s Oesophagus are also waiting beyond their planned dates for repeat surveillance endoscopy and are at risk of developing cancer without investigation.
“While the COVID-19 pandemic has aggravated the issues surrounding B.Oes and endoscopy, it has also fast tracked this change to diagnostic pathway”, said the Clinical lead in Scotland, Professor Grant Fullarton.
“Cytosponge helps specialist teams to triage Barrett’s Oesophagus and find which patients, with established pre-malignancy in the oesophagus, require endoscopy and treatment. We’ve already detected pre and early cancer in patients and early detection of this kind of cancer allows less invasive curative treatment involving endoscopic methods rather than major surgery.
"Such early diagnosis at scale across Scotland could revolutionise the outcome of what is now a poor outcome disease.”
More about the Cytosponge:
Cytosponge mobile diagnostic unit opens in England
How the Cytosponge works
BEST3 Cytosponge clinical trial identifies 10x more patients with Barrett's oesophagus than standard of care
Liz's story