Advanced Cancer Imaging
Every patient diagnosed with cancer will have imaging tests – X-rays, CT, MRI, ultrasound, PET - to help doctors understand its type, stage and response to treatment. This programme researches diagnostics advances, to understand the interior of the cancer cells at a molecular level. We seek to understand cancer cell death and metabolism, giving powerful information about efficacy of novel therapies as early as possible, in pre-clinical models and clinical trials. Selected talks by programme leads and members can be seen on our YouTube channel: https://www.youtube.com/playlist?list=PLU8zX5qFotyTWdTf7m5iT43sSAiLnx6qL
Twitter: @CRUKCamImaging https://twitter.com/CRUKCamImaging

The aim of the programme is to improve patient outcomes by advancing “personalised medicine” – making cancer treatment more effective by optimising treatment choices to the specific stage, type and molecular signature of the patient’s cancer, and based on earlier detection of treatment response. We also support the development of novel drug therapies via imaging biomarkers. The programme invests in infrastructure and staff for imaging projects across a range of modalities (MRI, CT, ultrasound and PET). Investments support the translation of new imaging techniques - from ideas to laboratory work and via patient studies to clinical use.
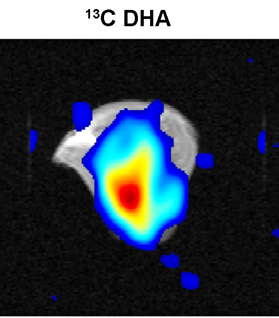
This is a new technique that increases the sensitivity of MRI by >10,000 times and which has allowed unprecedented studies of tissue metabolism in vivo. The method has been developed in collaboration with a commercial partner. Hyperpolarised carbon-13 imaging detects cellular metabolites involved in producing the energy that a cell needs to survive. We have shown that this allows the response of a tumour to be detected very early during treatment, which is important for evaluating the efficacy of new drugs in early stage clinical trials, and to find out, as quickly as possible, if individual patients are receiving the most effective combination and dose of drugs to treat their cancer. Cambridge is widely acknowledged as one of the world leaders in the clinical application of this revolutionary imaging technology to cancer. We have published results in breast and renal cancers.
https://pubmed.ncbi.nlm.nih.gov/34625424/
https://pubmed.ncbi.nlm.nih.gov/35053497/
We are running a study of hyperpolarised C13 imaging in patients with ovarian cancer, and supporting a trial of various drug therapies for renal cancer by imaging patients receiving different treatments. The programme supports personnel in Prof. Gallagher's group.
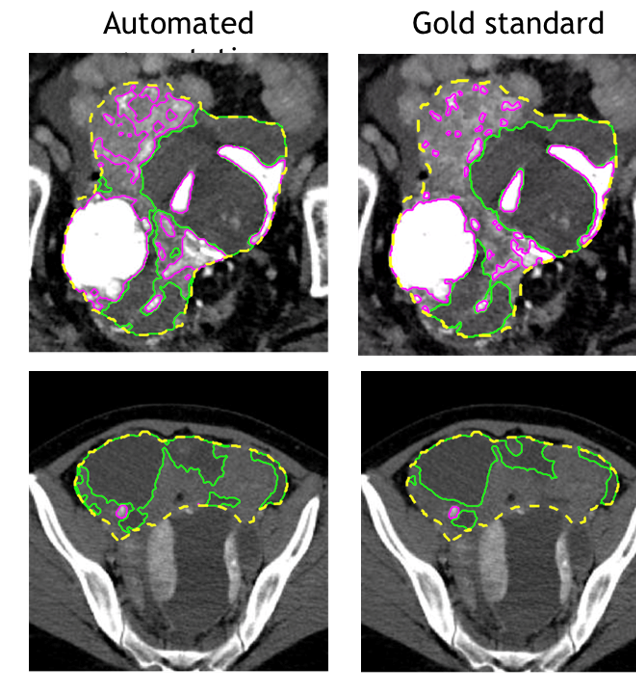
Currently, abdominal CT scans are evaluated visually and response is assessed based on unidimensional tumour measurements at different time points. Segmentation of the whole tumour burden is not performed routinely, as it is labour-intensive and also operator-dependent. Despite the recent advances in machine learning techniques, automated image segmentation for ovarian cancer is not yet available for clinical implementation owing to challenges including low tumour-to-tissue contrast and a lack of large-scale datasets for training and testing. The group has developed a prototype deep learning automated segmentation method for ovarian cancer on CT scans. The advanced cancer imaging programme is supporting these projects through investing in expert staffing.
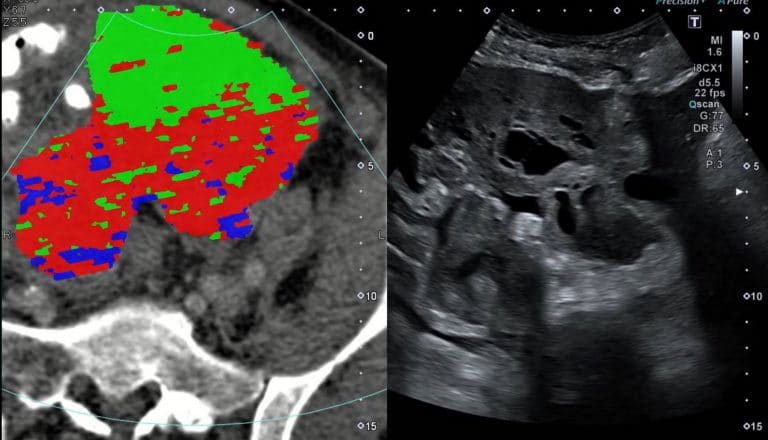
Assessment of the cellular composition of a tumour relies on biopsy, in which a needle is introduced into the tumour under ultrasound guidance to sample the tissue followed by histological analysis of the cells. The radiogenomics group is exploring novel methods to explore tumour cells using CT, then combine this analysis with the ultrasound images to guide the biopsy to ensure that different "habitats" within the tumour are all sampled. The ACI programme will be supporting input from an interventional radiologist as well as members of the radiomics group.
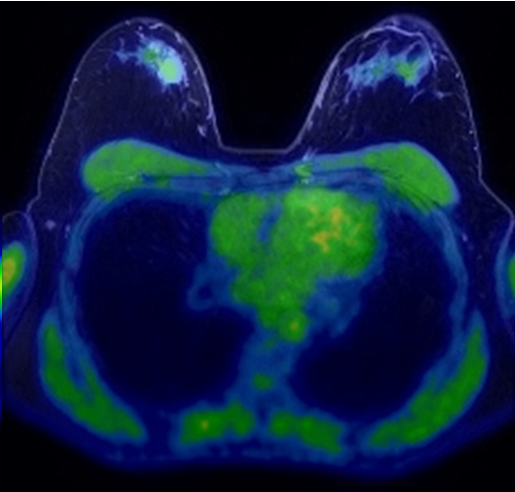
Cambridge opened the first PET/CT scanning department in the East of England in 2008. PET/CT technology uses two different imaging techniques – Positron Emission Tomography and Computed Tomography – and combines the results to produce detailed three-dimensional images of what is happening inside a patient's body. The combination allows both the structure and function of diseased areas to be studied. The Advanced Cancer Imaging programme is supporting the development of novel radionuclide-labelled agents, which are injected and then taken up by tumour cells. The agents emit positrons that can be detected and imaged. 18F-labelled C2AM is a novel agent that can be used to detect and image tumour cell death following treatment. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7726082/
Zr89-oregovomab is being developed as an agent for imaging ovarian tumour cells. Gallium-labelled radiotracers are also being researched.

We have a well-established programme of laboratory work, based in the Cambridge Institute, exploring cellular responses to therapies via imaging methods such as C2AM and hyper-polarised carbon-13. Published studies in ER+ breast cancer patient-derived xenografts showed that imaging with hyperpolarized [1-13C]pyruvate could be used to detect early responses to PI3K inhibitors and we plan to work with a local pharmaceutical partner to further this research.


















