DNA holds the instructions our cells need to grow, develop, divide and, eventually, even to die. This information is fragile and it can be damaged by toxic agents such as sunlight or chemicals (cigarettes, alcohol, etc.). Normally, our cells can cope with these hazards, but if the damage is not repaired correctly, it can cause a range of diseases including cancer.
A specific type of DNA damage known as inter-strand crosslinks are repaired by the orchestrated action of proteins of the so-called Fanconi anaemia (FA) pathway. The molecular mechanism of FA pathway activation has been a long-standing question. This step requires the attachment of ubiquitin, a 'protein tag', onto FANCD2. Intriguingly, FANCD2 seemed to be unable to accommodate this tag, due to its interaction with another protein, FANCI.
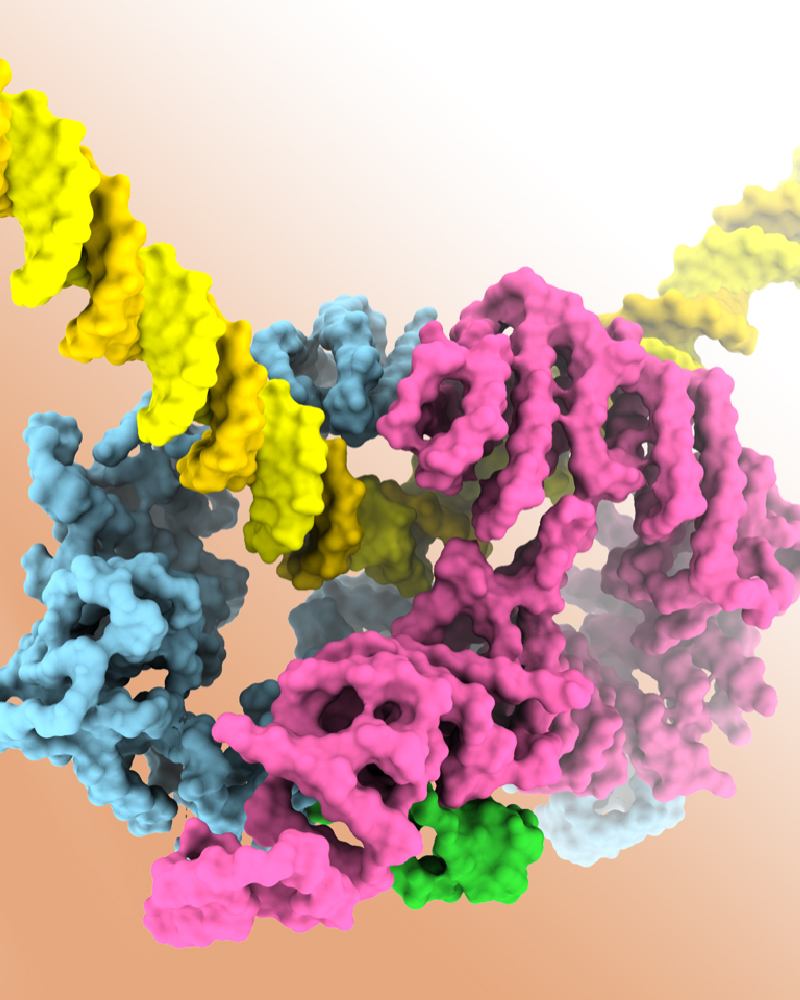
Using electron cryo-microscopy, researchers from Dr Lori Passmore's group at the MRC Laboratory of Molecular Biology have visualized the structure of the activated FANCD2-FANCI complex (D2I). The results show that DNA itself is the key element that permits the ubiquitination, and that D2I acts as a molecular clamp: D2I grasps DNA, and this reveals the specific site where ubiquitin is inserted. Interestingly, this work also suggests that the role of ubiquitin is not so much to signal the damage directly, but to tightly lock D2I onto the DNA. Then, D2I will recruit enzymes that will excise the damaged DNA, allowing its repair.















