Our genes are encoded in DNA but, first, must be copied into a temporary molecule – mRNA – before being translated into protein. A series of adenosines at one end of the mRNA, called the poly(A) tail, controls both the lifetime of the mRNA and how efficiently it is translated. Consequently, removal of the poly(A) tail regulates gene expression. Researchers from Dr Lori Passmore's group at the MRC Laboratory of Molecular Biology now show how RNA is recognised by some of the enzymes that shorten poly(A) tails.
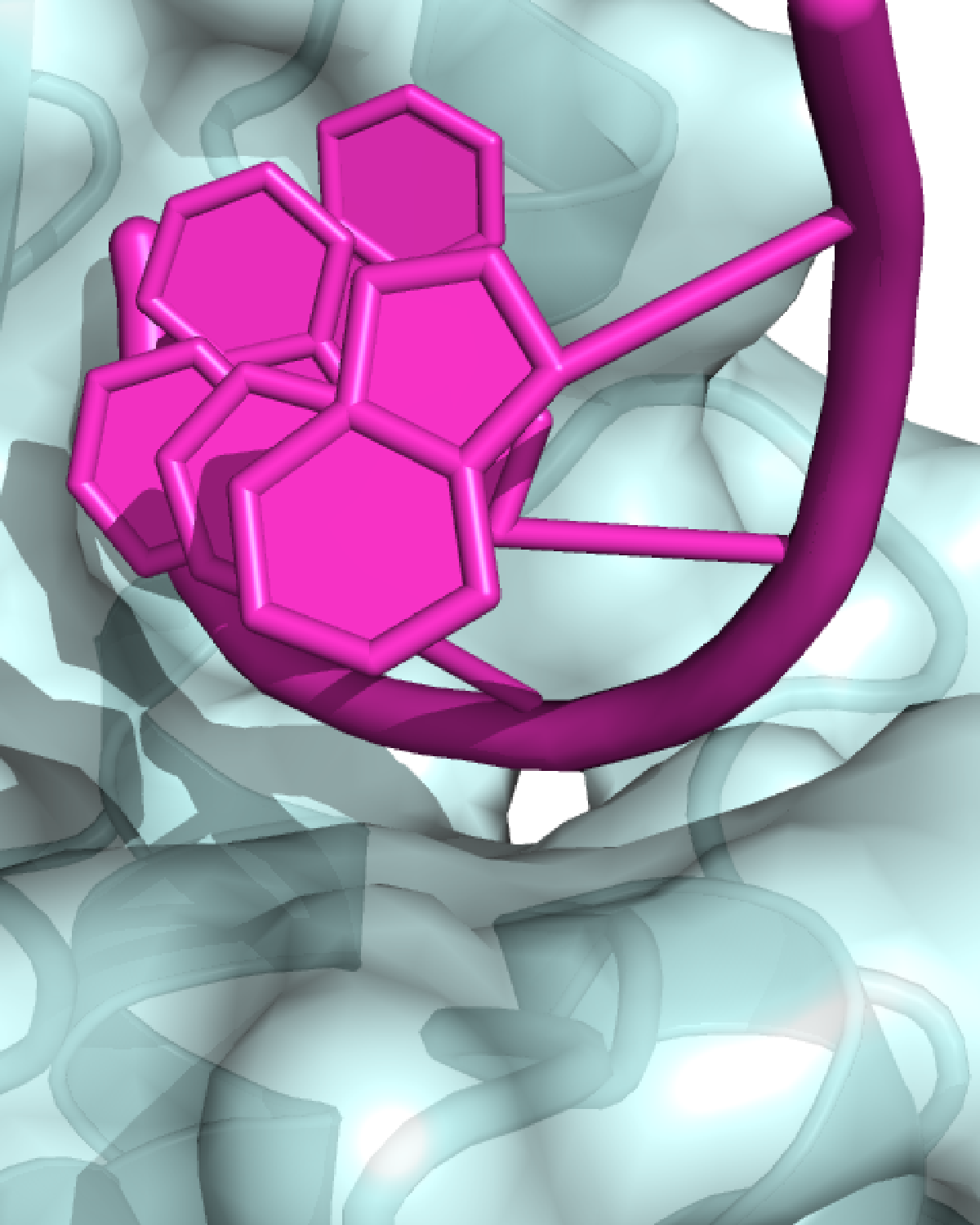
Single-stranded RNA is often thought to be primarily unstructured, but it has long been known that poly(A) in solution adopts a helical shape. Terence Tang, a PhD student in the Passmore lab, determined an X-ray crystal structure of one of the enzymes that shortens poly(A) tails and showed how it interacts with poly(A) RNA. Surprisingly, there were no canonical base-specific contacts between the protein and the RNA. Instead, the enzyme recognizes the intrinsic structure of poly(A). Thus, the protein is specific for poly(A) because it recognises the overall shape of the RNA, and not the individual bases.
This was a surprising finding since this unique feature of poly(A) RNA had not previously been shown to be recognised by proteins. This mode of protein-RNA interaction explains why these enzymes degrade only the poly(A) tail and not the mRNA itself. Since no other RNA base forms a similar structure, this provides a mechanistic basis for why poly(A) tails are unique.















