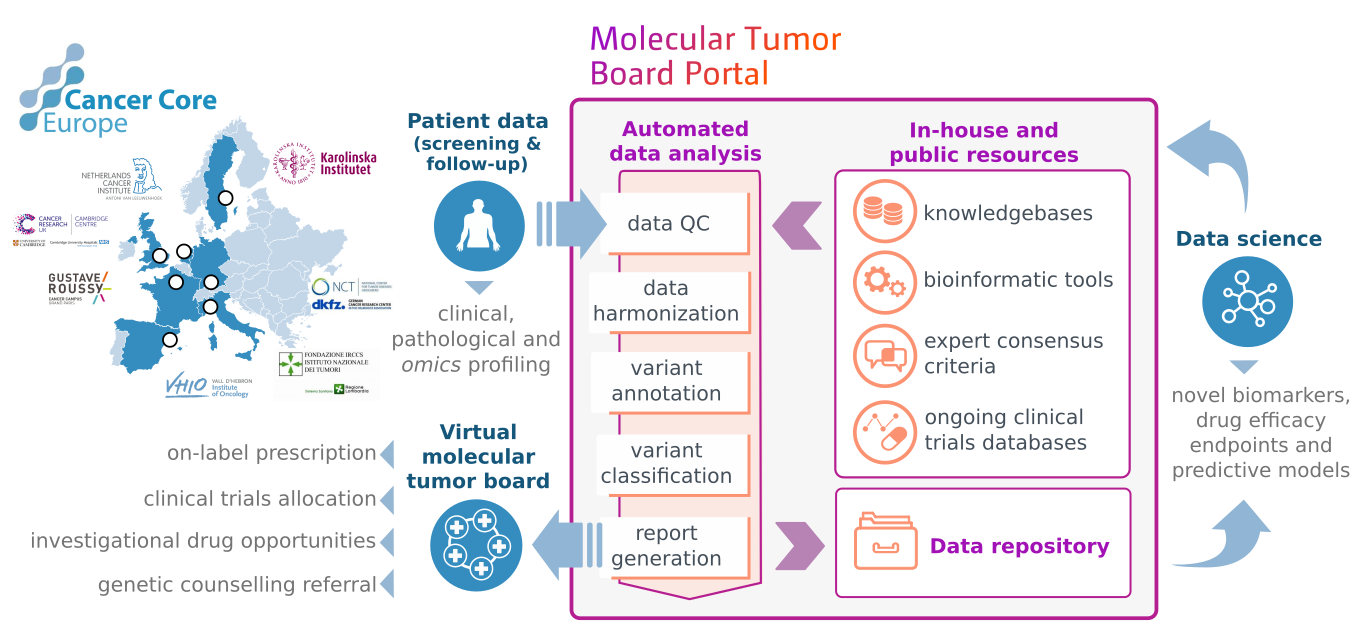
Cambridge is one of seven leading cancer centres across Europe that constitute Cancer Core Europe collaborating on a new type of clinical trial. Called the 'Basket of Baskets' trial, patients with different types of cancer are treated with the same targeted therapy because they share a common genetic mutation that’s causing their disease.
In order to select the best treatment for each patient signed up to the trial, the Cancer Core Europe centres have created a sophisticated digital platform to analyse large volumes of complex genetic and molecular data from patients’ tissue biopsies and blood tests. This is a world-first tool connecting centres across European countries.
An article published in Nature Medicine today describes the development and clinical use of the system, called the Molecular Tumour Board Portal (MTBP).
The MTBP system automatically collects and interprets large volumes of patient data, extracting the most clinically relevant information to produce an interactive data-rich online report.
Every patient report generated from the online clinical decision support system is discussed during one of the weekly web-based multi-disciplinary team meetings where members from all seven centres evaluate the results and agree on each patient’s personalised treatment plan.
The amount of data collected from cancer patients is increasing exponentially along with our understanding of which biomarkers determine how the disease will progress and which predict how tumours will respond to different treatments.
The MTBP tool has been developed to enable clinicians to make the best clinical decisions from the increasing volume and complexity of patient data by comparing it to existing databases and the latest published research on cancer biomarkers.
This means that personalised medicine is becoming a reality for more and more cancer patients, including Vera Webster, 71, who lives near Cambridge with her husband.
Vera works part-time as a book-keeper, plays badminton and enjoys spending time with her two sons and three grandchildren. She was diagnosed with endometrial cancer in 2017.
“I was treated with surgery and radiotherapy at Addenbrooke’s but the cancer returned 18 months later, this time to my lungs. After 6 cycles of chemotherapy, completed a year ago, the tumours had shrunk.”
“I started the Basket of Baskets trial in January 2020 in the light of the results I have received. I decided to join because research is always important and valuable for me and others taking part. I am happy to take part if it furthers progress.”
Vera’s consultant, Dr Richard Baird, from the Department of Oncology at the University of Cambridge and honorary consultant medical oncologist at CUHP, is the Cambridge principal investigator on the trial.
Professor Carlos Caldas, Cambridge lead for Cancer Core Europe and Director of the Breast Cancer Programme, CRUK Cambridge Centre said: “Many were sceptical that centres located in 7 different European countries could develop such a tool that works in practice every week. We are delighted to have pushed the boundaries and proven the unlikely is possible using modern web-based and intelligent tools such as the MTBP”.
Although designed and developed by Cancer Core Europe, the MTBP is also freely accessible to other cancer centres (https://mtbp.org).
So far data from over 500 cancer patients on the Basket of Baskets trial has been analysed. The trial demonstrates Cancer Core Europe can set up and run a complex cancer clinical trial as a truly collaborative process to accelerate access to innovative treatments for patients.
"The website automates data interpretation, which prevents errors associated with manual processing and allows systematic analysis based on a set of clinical criteria developed by experts," explains Dr David Tamborero, project lead from theScience for Life Laboratory, Karolinska Institutet.
"The use of this platform also avoids delays in the delivery of results, which is critical for patients whose condition may deteriorate rapidly."
The Cancer Core Europe collaboration brings together ground-breaking cancer researchers and clinicians from the Cancer Research UK Cambridge Centre (Cambridge), German Cancer Research Center & National Center for Tumor Diseases (Heidelberg), Institut Gustave Roussy (Paris), Karolinska Institutet (Stockholm), National Cancer Institute (Milan), Netherlands Cancer Institute (Amsterdam) and Vall d’Hebron Institute of Oncology (Barcelona).
Reference
Tamborero D., Dienstmann R. et al. Support systems to guide clinical decision-making in precision oncology: The Cancer Core Europe Molecular Tumor Board Portal Nature Medicine July 2020 https://dx.doi.org/10.1038/s41591-020-0969-2

















