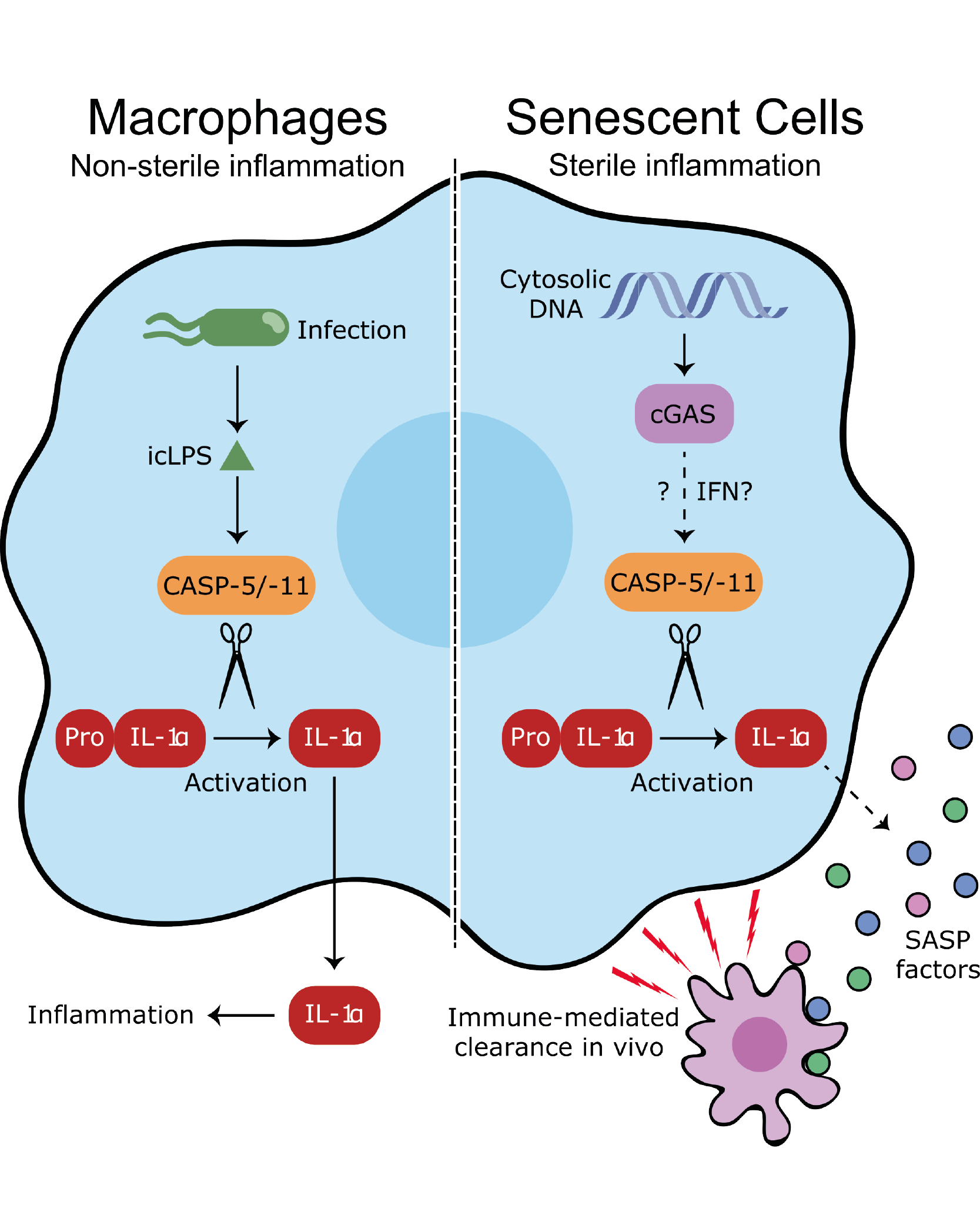
The immune system works tirelessly to prevent infection by microorganisms, but also removes trillions of our own aged and damaged cells daily. These complex processes are controlled by the exchange of information between cells through small proteins called cytokines. When genetic mutations that could cause cancer are detected in old or injured cells a programme called 'cellular senescence' is activated. This immediately stops the cell from proliferating and forming tumours, but also induces the secretion of multiple cytokines that direct the removal of senescent cells by the immune system. If senescent cells are not swiftly removed, new mutations lead to re-proliferation and cancer. One particular cytokine called interleukin-1 alpha (IL-1α) acts as the cornerstone of this process by directly controlling the secretion of most other cytokines from senescent cells.
A new publication from Dr Murray Clarke's group in the Cardiovascular Medicine Division of the Department of Medicine, in collaboration with Dr Masashi Narita's group at the CRUK Cambridge Institute, reveals how IL-1α is cleaved and activated by a protease called caspase-5, and that blocking this interaction prevents cytokine secretion leading to dangerous accumulation of senescent cells. Senescent cells that do not form tumours additionally accumulate throughout the body during natural aging, leading to harmful chronic inflammation driven by the same cytokines. Under these conditions, blocking caspase-5 may reduce the deleterious effects of these senescent cells, whilst retaining the immune system's ability to fight infections.















